Thermodynamics 101
This is useful, I promise
If you’re like me, and glaze over at theoretical math and physics, it can be hard to generate enthusiasm for caring about all those equations and laws and science stuff. What does this have to do with daily life, or making buildings, or living in buildings, or doing business?
Turns out, quite a lot - and now that I’ve taught college courses in building science, I’ve tried to help students see the connections between the theoretical and the practical, as it all plays out in buildings. I’m looking forward to bringing y’all some of that content in this and future newsletters!
Ok, let’s dive in.
First, a quick lesson in how thermodynamics work. You already know a lot of this, starting with the biggie: hot things always cool down, unless you intervene in some way - aka, hot flows to cold. So, a hot cup of coffee will become cool if you don’t put it in an insulated cup, to slow down its heat loss, or add more heat to it [put it in the microwave, say] to make up for that heat loss. Even then, eventually, it will still cool. How cool does it get? Well, it will get as cool as the ambient temperature around it, in other words, it won’t get colder than the air around it. If it did, then the “heat” from the ambient temperature would flow back into the coffee.
This is the 2nd Law of Thermodynamics: everything is seeking equilibrium. Think about our cup of coffee, and apply the same idea to a building. If you heat up the inside of the building on a cold winter day, it will gradually lose its heat to the exterior, unless you slow it down with insulation [like with did with the insulated coffee cup], and/or “add” more heat to the inside of the building [like we did by putting the coffee back in the microwave].
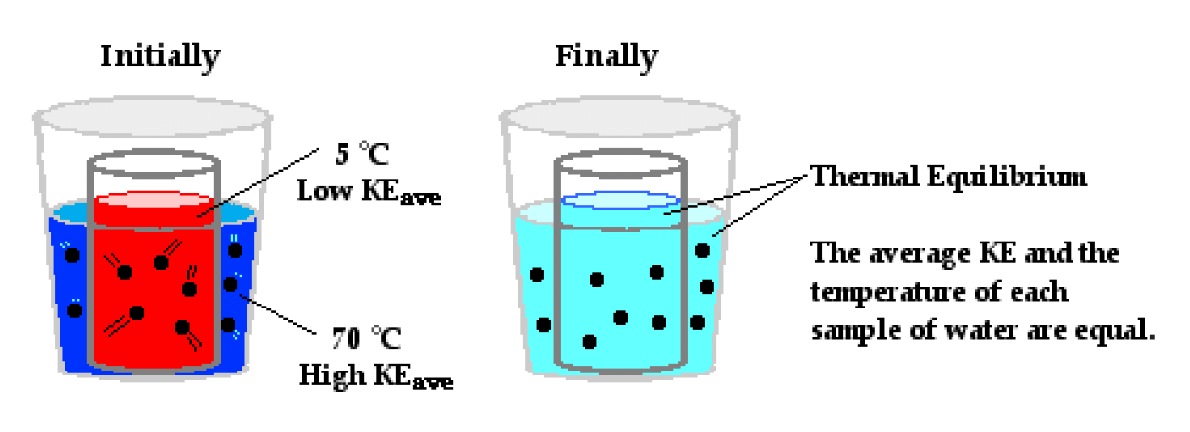
“Heat” is really just motion of molecules. If the molecules in a substance are moving faster, the substance will be “hotter.” So heat is a by-product of motion, which is energy. Energy never goes away, it just changes to forms that are deemed more or less useful by you. The energy in a cold lump of coal is not all that helpful, but release it using combustion, and you have the Industrial Revolution.
I’ll have to do another post on conservation of energy [thanks, Einstein], which will cause me to say all kinds of controversial things like “there’s no such thing as a net zero building.” Sacrilege, but true from a physics standpoint.
So anyway, back to thermodynamics - if anyone ever said to you, on a cold winter day, “close the door, you’re letting the cold in!” They were actually incorrect, thermodynamically speaking - what you were doing was letting the heat out…because heat always flows hot to cold [or, more accurately, heat flows from more to less - it seeks equilibrium].
In the summer, of course, the opposite would be true - if you opened your door, you’d be letting the heat in, not the cool air conditioned air out. Heat always flows from hotter to colder.
Knowing this helps you understand how all kinds of things work in a building. The most straightforward is insulation, which simply slows down the transfer of heat - it *resists* heat flow. Hence, when discussing the qualities of insulation, we use a metric called “R-value,” where the R stands for “resistance.” This is a building industry term, and not necessarily a scientific term, but the concepts still hold.
The higher the R-value, the more resistant that material is to heat flow. Everything has an R-value - clothing, air, water - but, R-value alone doesn’t tell you how “good” a material might be as an insulator. For example, you could take an insulation material that has a very high R-value, like closed cell spray foam insulation, whose R-value is 7 per inch, but if you install it incorrectly, or miss whole sections of the wall, then it’s not going to perform well.
Think of it like a winter coat. You could have the fanciest polar rated down parka, but if you don’t zip it up, it’s not going to work. All your heat will flow right out the gap at the front. Is it better than nothing? Sure. But would it be waaaay better if you zipped it up? Um, yes.
This is why even little gaps in insulation can have profound impact on the thermal performance of a building. And, when you factor in other real world conditions like wind, rain, people moving around inside, HVAC systems, etc, you’ve got quite a complicated scenario.
While it’s hard to mathematically map out all those scenarios, and to deeply understand all the physics at play, we can return to the big driver: our friend the 2nd Law of Thermodynamics, which says heat will flow to cold.
While we’re on that subject…thermodynamically, there is no such thing as “cold” - well, there is - 0 degrees kelvin, which is the death of the universe. Hot and cold are actually relative terms - not only in terms of science, but in terms of human comfort - just ask anyone who has had a thermostat battle with a partner or coworkers.
The idea of “thermal comfort” is fascinating, and has its roots in the capitalist pursuits of Willis Carrier, the inventor of air conditioning. We’ll get to that in a later post, but basically, he invented something no one thought they needed [air conditioning], then invented a whole set of charts and calculations to tell us that we’re uncomfortable unless we use his products [the psychrometric chart, which is now enshrined in most building codes].
And the idea of “comfort” itself is, some argue, an invented concept anyway. Read John E. Crowley’s “The Invention of Comfort: Sensibilities and Design in Early Modern Britain and Early America” for a taste of that idea.
Hot and cold are relative concepts from a thermodynamic perspective, and from a comfort perspective [back to our battling couple at the thermostat] - and once you understand that, you can not only design better spaces, you can also reduce the number of times a building occupant calls an owner with a complaint that the HVAC isn’t working.
There is SO much more to say about thermodynamics - we’ve barely scratched the surface here - but as you gain knowledge about these basic concepts, you can have more intelligent conversations with engineers, architects, installers, and maintenance folks - not because you’re quoting the 2nd Law, but because you understand the fundamentals. Mechanized HVAC is just moving air around to thwart the natural movement of hot to “cold,” and insulation is just putting your building in a winter parka and remembering to zip it up.
Thanks for reading! Your support means so much to me, truly. These posts come from years of field experience, teaching, and building, and I’m delighted to be able to pass that along.
Please consider sharing a subscription if you know someone who might be interested:

